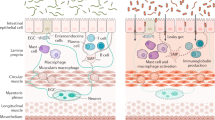
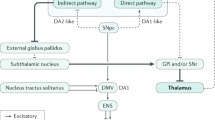
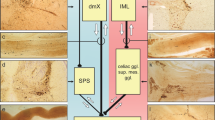
Key Points
-
The enteric nervous system (ENS) is the largest component of the autonomic nervous system and is uniquely equipped with intrinsic microcircuits that enable it to orchestrate gastrointestinal function independently of central nervous system (CNS) input
-
Because many neurotransmitters, signalling pathways and anatomical properties are common to the ENS and CNS, pathophysiological processes that underlie CNS disease often have enteric manifestations
-
Neuronal connections and the immune system might provide conduits that allow diseases acquired in the gut to spread to the brain
-
Transmissible spongiform encephalopathies, autistic spectrum disorders, Parkinson disease, Alzheimer disease, amyotrophic lateral sclerosis, and varicella zoster virus (VZV) infection are examples of disorders with both gastrointestinal and neurological consequences
-
VZV reactivations from latency in enteric and other autonomic neurons that lack cutaneous projections are occult causes of zoster without rash that lead to gastrointestinal disease, meningitis and strokes
-
Research on the gut–brain axis of disease is reasonably new, concepts are changing rapidly, and further investigation is much needed
Abstract
The enteric nervous system (ENS) is large, complex and uniquely able to orchestrate gastrointestinal behaviour independently of the central nervous system (CNS). An intact ENS is essential for life and ENS dysfunction is often linked to digestive disorders. The part the ENS plays in neurological disorders, as a portal or participant, has also become increasingly evident. ENS structure and neurochemistry resemble that of the CNS, therefore pathogenic mechanisms that give rise to CNS disorders might also lead to ENS dysfunction, and nerves that interconnect the ENS and CNS can be conduits for disease spread. We review evidence for ENS dysfunction in the aetiopathogenesis of autism spectrum disorder, amyotrophic lateral sclerosis, transmissible spongiform encephalopathies, Parkinson disease and Alzheimer disease. Animal models suggest that common pathophysiological mechanisms account for the frequency of gastrointestinal comorbidity in these conditions. Moreover, the neurotropic pathogen, varicella zoster virus (VZV), unexpectedly establishes latency in enteric and other autonomic neurons that do not innervate skin. VZV reactivation in these neurons produces no rash and is therefore a clandestine cause of gastrointestinal disease, meningitis and strokes. The gut–brain alliance has raised consciousness as a contributor to health, but a gut–brain axis that contributes to disease merits equal attention.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$209.00 per year
only $17.42 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout





Similar content being viewed by others
References
Gershon, M. D. The Second Brain (Harper Collins, 1998).
Furness, J. B., Callaghan, B. P., Rivera, L. R. & Cho, H. J. The enteric nervous system and gastrointestinal innervation: integrated local and central control. Adv. Exp. Med. Biol. 817, 39–71 (2014).
Clevers, H. The intestinal crypt, a prototype stem cell compartment. Cell 154, 274–284 (2013).
Turner, J. R. Intestinal mucosal barrier function in health and disease. Nat. Rev. Immunol. 9, 799–809 (2009).
Mowat, A. M. & Agace, W. W. Regional specialization within the intestinal immune system. Nat. Rev. Immunol. 14, 667–685 (2014).
Langley, J. N. The Autonomic Nervous System, Part 1 [1921] (Cornell Univ. Library, 2010).
Furness, J. B. The Enteric Nervous System (Blackwell Publishing, 2006).
Gershon, M. D. Developmental determinants of the independence and complexity of the enteric nervous system. Trends Neurosciences 33, 446–456 (2010).
Forsythe, P., Bienenstock, J. & Kunze, W. A. Vagal pathways for microbiome–brain–gut axis communication. Adv. Exp. Med. Biol. 817, 115–133 (2014).
Rush, A. J. et al. Vagus nerve stimulation (VNS) for treatment-resistant depressions: a multicenter study. Biol. Psychiatry 47, 276–286 (2000).
George, M. S. et al. Vagus nerve stimulation: a new tool for brain research and therapy. Biol. Psychiatry 47, 287–295 (2000).
Sampson, T. R. & Mazmanian, S. K. Control of brain development, function, and behavior by the microbiome. Cell Host Microbe 17, 565–576 (2015).
Yano, J. M. et al. Indigenous bacteria from the gut microbiota regulate host serotonin biosynthesis. Cell 161, 264–276 (2015).
Mayer, E. A., Knight, R., Mazmanian, S. K., Cryan, J. F. & Tillisch, K. Gut microbes and the brain: paradigm shift in neuroscience. J. Neurosci. 34, 15490–15496 (2014).
Tam, P. K. Hirschsprung's disease: a bridge for science and surgery. J. Pediatr. Surg. 51, 18–22 (2016).
Heuckeroth, R. O. Hirschsprung's disease, Down syndrome, and missing heritability: too much collagen slows migration. J. Clin. Invest. 125, 4323–4326 (2015).
Amiel, J. & Lyonnet, S. Hirschsprung disease, associated syndromes, and genetics: a review. J. Med. Genet. 38, 729–739 (2001).
Bern, C. Chagas' disease. N. Engl. J. Med. 373, 1882 (2015).
Avetisyan, M., Schill, E. M. & Heuckeroth, R. O. Building a second brain in the bowel. J. Clin. Invest. 125, 899–907 (2015).
Klingelhoefer, L. & Reichmann, H. Pathogenesis of Parkinson disease — the gut–brain axis and environmental factors. Nat. Rev. Neurol. 11, 625–636 (2015).
Collins, S. J., Lawson, V. A. & Masters, C. L. Transmissible spongiform encephalopathies. Lancet 363, 51–61 (2004).
Prusiner, S. B. Biology and genetics of prions causing neurodegeneration. Annu. Rev. Genet. 47, 601–623 (2013).
Aguzzi, A. Unraveling prion strains with cell biology and organic chemistry. Proc. Natl Acad. Sci. USA 105, 11–12 (2008).
Cronier, S. et al. Endogenous prion protein conversion is required for prion-induced neuritic alterations and neuronal death. FASEB J. 26, 3854–3861 (2012).
Ghosh, S. Mechanism of intestinal entry of infectious prion protein in the pathogenesis of variant Creutzfeldt–Jakob disease. Adv. Drug Deliv. Rev. 56, 915–920 (2004).
Kujala, P. et al. Prion uptake in the gut: identification of the first uptake and replication sites. PLoS Pathog. 7, e1002449 (2011).
Chiocchetti, R. et al. Anatomical evidence for ileal Peyer's patches innervation by enteric nervous system: a potential route for prion neuroinvasion? Cell Tissue Res. 332, 185–194 (2008).
Albanese, V. et al. Evidence for prion protein expression in enteroglial cells of the myenteric plexus of mouse intestine. Auton. Neurosci. 140, 17–23 (2008).
Martin, G. R. et al. Endogenous cellular prion protein regulates contractility of the mouse ileum. Neurogastroenterol. Motil. 24, e412–424 (2012).
Posar, A., Resca, F. & Visconti, P. Autism according to diagnostic and statistical manual of mental disorders 5th edition: the need for further improvements. J. Pediatr. Neurosci. 10, 146–148 (2015).
McElhanon, B. O., McCracken, C., Karpen, S. & Sharp, W. G. Gastrointestinal symptoms in autism spectrum disorder: a meta-analysis. Pediatrics 133, 872–883 (2014).
Halfon, N. & Kuo, A. A. What DSM-5 could mean to children with autism and their families. JAMA Pediatr. 167, 608–613 (2013).
Betancur, C. Etiological heterogeneity in autism spectrum disorders: more than 100 genetic and genomic disorders and still counting. Brain Res. 1380, 42–77 (2011).
Iossifov, I. et al. De novo gene disruptions in children on the autistic spectrum. Neuron 74, 285–299 (2012).
Neale, B. M. et al. Patterns and rates of exonic De novo mutations in autism spectrum disorders. Nature 485, 242–245 (2012).
O'Roak, B. J. et al. Sporadic autism exomes reveal a highly interconnected protein network of De novo mutations. Nature 485, 246–250 (2012).
O'Roak, B. J. et al. Multiplex targeted sequencing identifies recurrently mutated genes in autism spectrum disorders. Science 338, 1619–1622 (2012).
Schulze, T. G. & McMahon, F. J. Defining the phenotype in human genetic studies: forward genetics and reverse phenotyping. Hum. Hered. 58, 131–138 (2004).
Bernier, R. et al. Disruptive CHD8 mutations define a subtype of autism early in development. Cell 158, 263–276 (2014).
Sweatt, J. D. Pitt–Hopkins syndrome: intellectual disability due to loss of TCF4-regulated gene transcription. Exp. Mol. Med. 45, e21 (2013).
Grubisic, V., Kennedy, A. J., Sweatt, J. D. & Parpura, V. Pitt–Hopkins mouse model has altered particular gastrointestinal transits in vivo. Autism Res. 8, 629–633 (2015).
Marler, S. et al. Brief report: whole blood serotonin levels and gastrointestinal symptoms in autism spectrum disorder. J. Autism Dev. Disord. 46, 1124–1130 (2016).
Matondo, R. B. et al. Deletion of the serotonin transporter in rats disturbs serotonin homeostasis without impairing liver regeneration. Am. J. Physiol. Gastrointest. Liver Physiol. 296, G963–G968 (2009).
Morrissey, J. J., Walker, M. N. & Lovenberg, W. The absence of tryptophan hydroxylase activity in blood platelets. Proc. Soc. Exp. Biol. Med. 154, 496–499 (1977).
Lesch, K. P., Wolozin, B. L., Murphy, D. L. & Riederer, P. Primary structure of the human platelet serotonin (5-HT) uptake site: identity with the brain 5-HT transporter. J. Neurochem. 60, 2319–2322 (1993).
Veenstra-VanderWeele, J. et al. Autism gene variant causes hyperserotonemia, serotonin receptor hypersensitivity, social impairment and repetitive behavior. Proc. Natl Acad. Sci. USA 109, 5469–5474 (2012).
Margolis, K. G. et al. Serotonin transporter variant drives preventable gastrointestinal abnormalities in development and function. J. Clin. Invest. 126, 2221–2235 (2016).
Bromley, R. L. et al. The prevalence of neurodevelopmental disorders in children prenatally exposed to antiepileptic drugs. J. Neurol. Neurosurg. Psychiatry 84, 637–643 (2013).
Christensen, J. et al. Prenatal valproate exposure and risk of autism spectrum disorders and childhood autism. JAMA 309, 1696–1703 (2013).
Roullet, F. I., Lai, J. K. & Foster, J. A. In utero exposure to valproic acid and autism — a current review of clinical and animal studies. Neurotoxicol. Teratol. 36, 47–56 (2013).
de Theije, C. G. et al. Intestinal inflammation in a murine model of autism spectrum disorders. Brain Behav. Immun. 37, 240–247 (2014).
Ghia, J. E. et al. Serotonin has a key role in pathogenesis of experimental colitis. Gastroenterology 137, 1649–1660 (2009).
Haub, S. et al. Enhancement of intestinal inflammation in mice lacking interleukin 10 by deletion of the serotonin reuptake transporter. Neurogastroenterol. Motil. 22, 826–e229 (2010).
Bischoff, S. C. et al. Role of serotonin in intestinal inflammation: knockout of serotonin reuptake transporter exacerbates 2,4,6-trinitrobenzene sulfonic acid colitis in mice. Am. J. Physiol. Gastrointest Liver Physiol. 296, G685–G695 (2009).
Gershon, M. D. Serotonin is a sword and a shield of the bowel: serotonin plays offense and defense. Trans. Am. Clin. Climatol. Assoc. 123, 268–280; discussion 280 (2012).
Gershon, M. D. 5-Hydroxytryptamine (serotonin) in the gastrointestinal tract. Curr. Opin. Endocrinol. Diabetes Obes. 20, 14–21 (2013).
Liu, Z., Li, N. & Neu, J. Tight junctions, leaky intestines, and pediatric diseases. Acta Paediatr. 94, 386–393 (2005).
D'Eufemia, P. et al. Abnormal intestinal permeability in children with autism. Acta Paediatr. 85, 1076–1079 (1996).
Robertson, M. A. et al. Intestinal permeability and glucagon-like peptide-2 in children with autism: a controlled pilot study. J. Autism Dev. Disord. 38, 1066–1071 (2008).
de Magistris, L. et al. Alterations of the intestinal barrier in patients with autism spectrum disorders and in their first-degree relatives. J. Pediatr. Gastroenterol. Nutr. 51, 418–424 (2010).
Malkova, N. V., Yu, C. Z., Hsiao, E. Y., Moore, M. J. & Patterson, P. H. Maternal immune activation yields offspring displaying mouse versions of the three core symptoms of autism. Brain Behav. Immun. 26, 607–616 (2012).
Hsiao, E. Y. et al. Microbiota modulate behavioral and physiological abnormalities associated with neurodevelopmental disorders. Cell 155, 1451–1463 (2013).
Neunlist, M. et al. The digestive neuronal–glial–epithelial unit: a new actor in gut health and disease. Nat. Rev. Gastroenterol. Hepatol. 10, 90–100 (2013).
Jankovic, J. Parkinson's disease: clinical features and diagnosis. J. Neurol. Neurosurg. Psychiatry 79, 368–376 (2008).
Qualman, S. J., Haupt, H. M., Yang, P. & Hamilton, S. R. Esophageal Lewy bodies associated with ganglion cell loss in achalasia. Similarity to Parkinson's disease. Gastroenterology 87, 848–856 (1984).
Kupsky, W. J., Grimes, M. M., Sweeting, J., Bertsch, R. & Cote, L. J. Parkinson's disease and megacolon: concentric hyaline inclusions (Lewy bodies) in enteric ganglion cells. Neurology 37, 1253–1255 (1987).
Wakabayashi, K., Takahashi, H., Takeda, S., Ohama, E. & Ikuta, F. Parkinson's disease: the presence of Lewy bodies in Auerbach's and Meissner's plexuses. Acta Neuropathol. (Berl.) 76, 217–221 (1988).
Wakabayashi, K., Takahashi, H., Ohama, E. & Ikuta, F. Tyrosine hydroxylase-immunoreactive intrinsic neurons in the Auerbach's and Meissner's plexuses of humans. Neurosci. Lett. 96, 259–263 (1989).
Li, Z. S., Pham, T. D., Tamir, H., Chen, J. J. & Gershon, M. D. Enteric dopaminergic neurons: definition, developmental lineage, and effects of extrinsic denervation. J. Neurosci. 24, 1330–1339 (2004).
Li, Z. S., Schmauss, C., Cuenca, A., Ratcliffe, E. & Gershon, M. D. Physiological modulation of intestinal motility by enteric dopaminergic neurons and the D2 receptor: analysis of dopamine receptor expression, location, development, and function in wild-type and knock-out mice. J. Neurosci. 26, 2798–2807 (2006).
Tieu, K. A guide to neurotoxic animal models of Parkinson's disease. Cold Spring Harb. Perspect. Med. 1, a009316 (2011).
Anderson, G. et al. Loss of enteric dopaminergic neurons and associated changes in colon motility in an MPTP mouse model of Parkinson's disease. Exp. Neurol. 207, 4–12 (2007).
Chandra, S., Gallardo, G., Fernandez-Chacon, R., Schluter, O. M. & Sudhof, T. C. α-Synuclein cooperates with CSPα in preventing neurodegeneration. Cell 123, 383–396 (2005).
Westphal, C. H. & Chandra, S. S. Monomeric synucleins generate membrane curvature. J. Biol. Chem. 288, 1829–1840 (2013).
Vargas, K. J. et al. Synucleins regulate the kinetics of synaptic vesicle endocytosis. J. Neurosci. 34, 9364–9376 (2014).
Braak, H. & Braak, E. Pathoanatomy of Parkinson's disease. J. Neurol. 247 (Suppl. 2), II/3–II/10 (2000).
Preterre, C. et al. Optimizing Western Blots for the detection of endogenous α-synuclein in the enteric nervous system. J. Parkinsons Dis. 5, 765–772 (2015).
Aldecoa, I. et al. Alpha-synuclein immunoreactivity patterns in the enteric nervous system. Neurosci. Lett. 602, 145–149 (2015).
Miraglia, F., Betti, L., Palego, L. & Giannaccini, G. Parkinson's disease and alpha-synucleinopathies: from arising pathways to therapeutic challenge. Cent. Nerv. Syst. Agents Med. Chem. 15, 109–116 (2015).
Hallett, P. J., McLean, J. R., Kartunen, A., Langston, J. W. & Isacson, O. Alpha-synuclein overexpressing transgenic mice show internal organ pathology and autonomic deficits. Neurobiol. Dis. 47, 258–267 (2012).
Wang, L., Fleming, S. M., Chesselet, M. F. & Tache, Y. Abnormal colonic motility in mice overexpressing human wild-type α-synuclein. Neuroreport 19, 873–876 (2008).
Wang, L. et al. Mice overexpressing wild-type human α-synuclein display alterations in colonic myenteric ganglia and defecation. Neurogastroenterol. Motil. 24, e425–e436 (2012).
Polymeropoulos, M. H. et al. Mutation in the α-synuclein gene identified in families with Parkinson's disease. Science 276, 2045–2047 (1997).
Kruger, R. et al. Ala30Pro mutation in the gene encoding α-synuclein in Parkinson's disease. Nat. Genet. 18, 106–108 (1998).
Kuo, Y. M. et al. Extensive enteric nervous system abnormalities in mice transgenic for artificial chromosomes containing Parkinson disease-associated α-synuclein gene mutations precede central nervous system changes. Hum. Mol. Genet. 19, 1633–1650 (2010).
Lebouvier, T. et al. Routine colonic biopsies as a new tool to study the enteric nervous system in living patients. Neurogastroenterol. Motil. 22, e11–e14 (2010).
Gold, A., Turkalp, Z. T. & Munoz, D. G. Enteric alpha-synuclein expression is increased in Parkinson's disease but not Alzheimer's disease. Mov. Disord. 28, 237–240 (2013).
US Preventive Services Task Force. Screening for colorectal cancer: U.S. Preventive Services Task Force recommendation statement. JAMA 315, 2564-2575 (2016).
Hilton, D. et al. Accumulation of α-synuclein in the bowel of patients in the pre-clinical phase of Parkinson's disease. Acta Neuropathol. 127, 235–241 (2014).
Shannon, K. M. et al. Alpha-synuclein in colonic submucosa in early untreated Parkinson's disease. Mov. Disord. 27, 709–715 (2012).
Visanji, N. P. et al. Colonic mucosal α-synuclein lacks specificity as a biomarker for Parkinson disease. Neurology 84, 609–616 (2015).
Stokholm, M. G., Danielsen, E. H., Hamilton-Dutoit, S. J. & Borghammer, P. Pathological α-synuclein in gastrointestinal tissues from prodromal Parkinson disease patients. Ann. Neurol. 79, 940–949 (2016).
Singaram, C. et al. Dopaminergic defect of enteric nervous system in Parkinson's disease patients with chronic constipation. Lancet 346, 861–864 (1995).
Annerino, D. M. et al. Parkinson's disease is not associated with gastrointestinal myenteric ganglion neuron loss. Acta Neuropathol. 124, 665–680 (2012).
Pickel, V. M., Beckley, S. C., Joh, T. H. & Reis, D. J. Ultrastructural immunocytochemical localization of tyrosine hydroxylase in the neostriatum. Brain Res. 225, 373–385 (1981).
Weiner, N. Regulation of norepinephrine biosynthesis. Annu. Rev. Pharmacol. 10, 273–290 (1970).
Braak, E. et al. alpha-synuclein immunopositive Parkinson's disease-related inclusion bodies in lower brain stem nuclei. Acta Neuropathol. 101, 195–201 (2001).
Del Tredici, K., Rub, U., De Vos, R. A., Bohl, J. R. & Braak, H. Where does parkinson disease pathology begin in the brain? J. Neuropathol. Exp. Neurol. 61, 413–426 (2002).
Braak, H., Rub, U., Gai, W. P. & Del Tredici, K. Idiopathic Parkinson's disease: possible routes by which vulnerable neuronal types may be subject to neuroinvasion by an unknown pathogen. J. Neural Transm. 110, 517–536 (2003).
Del Tredici, K. & Duda, J. E. Peripheral Lewy body pathology in Parkinson's disease and incidental Lewy body disease: four cases. J. Neurol. Sci. 310, 100–106 (2011).
Phillips, R. J., Walter, G. C., Wilder, S. L., Baronowsky, E. A. & Powley, T. L. Alpha-synuclein-immunopositive myenteric neurons and vagal preganglionic terminals: autonomic pathway implicated in Parkinson's disease? Neuroscience 153, 733–750 (2008).
Gorell, J. M., Johnson, C. C., Rybicki, B. A., Peterson, E. L. & Richardson, R. J. The risk of Parkinson's disease with exposure to pesticides, farming, well water, and rural living. Neurology 50, 1346–1350 (1998).
Pan-Montojo, F. et al. Progression of Parkinson's disease pathology is reproduced by intragastric administration of rotenone in mice. PLoS ONE 5, e8762 (2010).
Pan-Montojo, F. et al. Environmental toxins trigger PD-like progression via increased alpha-synuclein release from enteric neurons in mice. Sci. Rep. 2, 898 (2012).
Ulusoy, A. et al. Caudo-rostral brain spreading of α-synuclein through vagal connections. EMBO Mol. Med. 5, 1051–1059 (2013).
Holmqvist, S. et al. Direct evidence of Parkinson pathology spread from the gastrointestinal tract to the brain in rats. Acta Neuropathol. 128, 805–820 (2014).
Svensson, E. et al. Vagotomy and subsequent risk of Parkinson's disease. Ann. Neurol. 78, 522–529 (2015).
Tysnes, O. B. et al. Does vagotomy reduce the risk of Parkinson's disease? Ann. Neurol. 78, 1011–1012 (2015).
Jellinger, K. A. A critical evaluation of current staging of α-synuclein pathology in Lewy body disorders. Biochim. Biophys. Acta 1792, 730–740 (2009).
Kalaitzakis, M. E., Graeber, M. B., Gentleman, S. M. & Pearce, R. K. The dorsal motor nucleus of the vagus is not an obligatory trigger site of Parkinson's disease: a critical analysis of α-synuclein staging. Neuropathol. Appl. Neurobiol. 34, 284–295 (2008).
Kingsbury, A. E. et al. Brain stem pathology in Parkinson's disease: an evaluation of the Braak staging model. Mov. Disord. 25, 2508–2515 (2010).
Ubhi, K. & Masliah, E. Alzheimer's disease: recent advances and future perspectives. J. Alzheimers Dis. 33, S185–S194 (2013).
Schliebs, R. Basal forebrain cholinergic dysfunction in Alzheimer's disease — interrelationship with beta-amyloid, inflammation and neurotrophin signaling. Neurochem. Res. 30, 895–908 (2005).
Arai, H. et al. Expression patterns of beta-amyloid precursor protein (β-APP) in neural and nonneural human tissues from Alzheimer's disease and control subjects. Ann. Neurol. 30, 686–693 (1991).
Puig, K. L., Swigost, A. J., Zhou, X., Sens, M. A. & Combs, C. K. Amyloid precursor protein expression modulates intestine immune phenotype. J. Neuroimmune Pharmacol. 7, 215–230 (2012).
Semar, S. et al. Changes of the enteric nervous system in amyloid-β protein precursor transgenic mice correlate with disease progression. J. Alzheimers Dis. 36, 7–20 (2013).
Puig, K. L. et al. Overexpression of mutant amyloid-β protein precursor and presenilin 1 modulates enteric nervous system. J. Alzheimers Dis. 44, 1263–1278 (2015).
Joachim, C. L., Mori, H. & Selkoe, D. J. Amyloid β-protein deposition in tissues other than brain in Alzheimer's disease. Nature 341, 226–230 (1989).
Deguchi, E., Iwai, N., Goto, Y., Yanagihara, J. & Fushiki, S. An immunohistochemical study of neurofilament and microtubule-associated Tau protein in the enteric innervation in Hirschsprung's disease. J. Pediatr. Surg. 28, 886–890 (1993).
Tam, P. K. & Owen, G. An immunohistochemical study of neuronal microtubule-associated proteins in Hirschsprung's disease. Hum. Pathol. 24, 424–431 (1993).
Phillips, R. J., Walter, G. C., Ringer, B. E., Higgs, K. M. & Powley, T. L. Alpha-synuclein immunopositive aggregates in the myenteric plexus of the aging Fischer 344 rat. Exp. Neurol. 220, 109–119 (2009).
Shankle, W. R. et al. Studies of the enteric nervous system in Alzheimer disease and other dementias of the elderly: enteric neurons in Alzheimer disease. Mod. Pathol. 6, 10–14 (1993).
Jovicic, A., Paul, J. W. 3rd & Gitler, A. D. Nuclear transport dysfunction: a common theme in amyotrophic lateral sclerosis and frontotemporal dementia. J. Neurochem. http://dx.doi.org/10.1111/jnc.13642 (2016).
Geser, F., Martinez-Lage, M., Kwong, L. K., Lee, V. M. & Trojanowski, J. Q. Amyotrophic lateral sclerosis, frontotemporal dementia and beyond: the TDP-43 diseases. J. Neurol. 256, 1205–1214 (2009).
Kabashi, E. et al. TARDBP mutations in individuals with sporadic and familial amyotrophic lateral sclerosis. Nat. Genet. 40, 572–574 (2008).
Sreedharan, J. et al. TDP-43 mutations in familial and sporadic amyotrophic lateral sclerosis. Science 319, 1668–1672 (2008).
Chio, A. et al. Extensive genetics of ALS: a population-based study in Italy. Neurology 79, 1983–1989 (2012).
Neumann, M. et al. Ubiquitinated TDP-43 in frontotemporal lobar degeneration and amyotrophic lateral sclerosis. Science 314, 130–133 (2006).
Wegorzewska, I., Bell, S., Cairns, N. J., Miller, T. M. & Baloh, R. H. TDP-43 mutant transgenic mice develop features of ALS and frontotemporal lobar degeneration. Proc. Natl Acad. Sci. USA 106, 18809–18814 (2009).
Guo, Y. et al. HO-1 induction in motor cortex and intestinal dysfunction in TDP-43 A315T transgenic mice. Brain Res. 1460, 88–95 (2012).
Esmaeili, M. A., Panahi, M., Yadav, S., Hennings, L. & Kiaei, M. Premature death of TDP-43 (A315T) transgenic mice due to gastrointestinal complications prior to development of full neurological symptoms of amyotrophic lateral sclerosis. Int. J. Exp. Pathol. 94, 56–64 (2013).
Herdewyn, S. et al. Prevention of intestinal obstruction reveals progressive neurodegeneration in mutant TDP-43 (A315T) mice. Mol. Neurodegener. 9, 24 (2014).
Hatzipetros, T. et al. C57BL/6J congenic Prp-TDP43A315T mice develop progressive neurodegeneration in the myenteric plexus of the colon without exhibiting key features of ALS. Brain Res. 1584, 59–72 (2014).
Kaur, S. J., McKeown, S. R. & Rashid, S. Mutant SOD1 mediated pathogenesis of amyotrophic lateral sclerosis. Gene 577, 109–118 (2016).
Wu, S., Yi, J., Zhang, Y. G., Zhou, J. & Sun, J. Leaky intestine and impaired microbiome in an amyotrophic lateral sclerosis mouse model. Physiol. Rep. 3, e12356 (2015).
Gross, E. R., Gershon, M. D., Margolis, K. G., Gertsberg, Z. V. & Cowles, R. A. Neuronal serotonin regulates growth of the intestinal mucosa in mice. Gastroenterology 143, 408–417.e2 (2012).
Natale, G., Pasquali, L., Paparelli, A. & Fornai, F. Parallel manifestations of neuropathologies in the enteric and central nervous systems. Neurogastroenterol. Motil. 23, 1056–1065 (2011).
Colby, D. W. & Prusiner, S. B. Prions. Cold Spring Harb. Perspect. Biol. 3, a006833 (2011).
Pinkas, A. & Aschner, M. Advanced glycation end-products and their receptors: related pathologies, recent therapeutic strategies, and a potential model for future neurodegeneration studies. Chem. Res. Toxicol. 29, 707–714 (2016).
Deng, H., Gao, K. & Jankovic, J. The role of FUS gene variants in neurodegenerative diseases. Nat. Rev. Neurol. 10, 337–348 (2014).
Sharma, A. et al. ALS-associated mutant FUS induces selective motor neuron degeneration through toxic gain of function. Nat. Commun. 7, 10465 (2016).
Parakh, S. & Atkin, J. D. Protein folding alterations in amyotrophic lateral sclerosis. Brain Res. http://dx.doi.org/10.1016/j.brainres.2016.04.010 (2016).
Gershon, A. A. et al. Varicella zoster virus infection. Nat. Rev. Dis. Primers 1, 15016 (2015).
Gershon, A. A., Chen, J. & Gershon, M. D. Use of saliva to identify varicella zoster virus infection of the gut. Clin. Infect. Dis. 61, 536–544 (2015).
Gershon, A. A. et al. Latency of varicella zoster virus in dorsal root, cranial, and enteric ganglia in vaccinated children. Trans. Am. Clin. Climatol. Assoc. 123, 17–33; discussion 33–35 (2012).
Holland-Cunz, S. et al. Acquired intestinal aganglionosis after a lytic infection with varicella-zoster virus. J. Pediatr. Surg. 41, e29–e31 (2006).
Levin, M. J. Varicella-zoster virus and virus DNA in the blood and oropharynx of people with latent or active varicella-zoster virus infections. J. Clin. Virol. 61, 487–495 (2014).
Edelman, D. A. et al. Ogilvie syndrome and herpes zoster: case report and review of the literature. J. Emerg. Med. 39, 696–700 (2009).
Mehta, S. K. et al. Varicella-zoster virus in the saliva of patients with herpes zoster. J. Infect. Dis. 197, 654–657 (2008).
Duncan, C. J. & Hambleton, S. Varicella zoster virus immunity: a primer. J. Infect. 71, S47–S53 (2015).
Johnson, B. H. et al. Annual incidence rates of herpes zoster among an immunocompetent population in the United States. BMC Infect. Dis. 15, 502 (2015).
Acknowledgements
M.R. receives research support from Ivan and Phyllis Seidenberg, the Paul Marks Scholars Program, and the American Gastroenterological Association – Takeda Pharmaceuticals International Research Scholar Award in Neurogastroenterology. M.D.G. is supported by grant NS15547 from the NIH and the Einhorn Family Charitable Trust.
Author information
Authors and Affiliations
Contributions
The authors contributed equally to the review.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing financial interests.
Rights and permissions
About this article
Cite this article
Rao, M., Gershon, M. The bowel and beyond: the enteric nervous system in neurological disorders. Nat Rev Gastroenterol Hepatol 13, 517–528 (2016). https://doi.org/10.1038/nrgastro.2016.107
Published:
Issue Date:
DOI: https://doi.org/10.1038/nrgastro.2016.107
This article is cited by
-
The nerve cells in gastrointestinal cancers: from molecular mechanisms to clinical intervention
Oncogene (2024)
-
Assessment of machine learning strategies for simplified detection of autism spectrum disorder based on the gut microbiome composition
Neural Computing and Applications (2024)
-
Diet–gut microbiome interaction and ferulic acid bioavailability: implications on neurodegenerative disorders
European Journal of Nutrition (2024)
-
Gut microbiota and type 2 diabetes mellitus: a focus on the gut-brain axis
Endocrine (2024)
-
The mechanisms of nerve injury caused by viral infection in the occurrence of gastrointestinal motility disorder-related diseases
Virology Journal (2023)